Research fields
1) Targeting bacterial enzymes and human lectins for discovery of antibacterial agents
Infections caused by antibiotic-resistant bacteria continue to challenge health-care systems worldwide. We face growing resistance of Gram-positive and Gram-negative pathogens that cause infections in hospitals and in the community, with the so-called antibiotic-resistant ‘superbugs’ that now represent a major global health problem. Research at the Chair of pharmaceutical chemistry has led to a number of important peptidoglycan (PG) biosynthesis inhibitors, including inhibitors of the MurA-MurF enzymes, D-Ala-D-Ala ligase B, aspartate ligase, transpeptidase and transglycosylase domain inhibitors of penicillin-binding proteins, inhibitors of DNA gyrase and topoisomerase IV, the two enzymes involved in modulation of DNA topology during replication, and inhibitors of Mycobacterium tuberculosis enoyl acyl carrier protein reductase (InhA) that facilitates reduction of long chain fatty acids. Research is also focused on discovery of novel antagonists of lectin FimH, which is a key virulence factor in urinary tract infections caused by Escherichia coli.
Enzymes involved in peptidoglycan biosynthesis
Project leader: prof. dr. Stanislav Gobec
Description: Peptidoglycan is a macromolecule that is essential for and specific to the bacterial cell wall. The enzymes involved in its biosynthetic pathway constitute potential targets for the discovery of new antibiotics. During the past 15 years, researchers at the Chair of pharmaceutical chemistry have developed a number of important peptidoglycan biosynthesis inhibitors, the most important of which are inhibitors of the Mur enzymes (MurA to MurF) and D-Ala-D-Ala ligase B, and of penicillin-binding proteins. Currently, we are optimising their on-target activities, phys-chem and ADMET properties. The optimisation of inhibitors is following iterative approach including design, synthesis of analogues, biochemical, structural, microbiological and preliminary pharmacokinetic and toxicological evaluation.
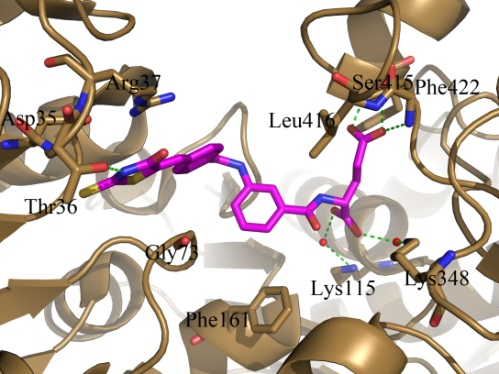
Crystal structure of MurD-inhibitor complex obtained during structure-based design of novel inhibitors of intracellular steps of peptidoglycan biosynthesis.
Recent publications:
1. Patin Delphine, Turk Samo, Barreteau Hélène, Mainardi Jean-Luc, Arthur Michel, Gobec Stanislav, Mengin-Lecreulx Dominique, Blanot Didier. Unusual substrate specificity of the peptidoglycan MurE ligase from Erysipelothrix rhusiopathiae. Biochimie 2016, 121, 209-218, doi: 10.1016/j.biochi.2015.12.006.
2. Šink Roman, Kotnik Miha, Zega Anamarija, Barreteau Hélène, Gobec Stanislav, Blanot Didier, Dessen Andréa, Contreras-Martel Carlos. Crystallographic study of peptidoglycan biosynthesis enzyme MurD: domain movement revisited. PloS One 2016,11, 3, doi: 10.1371/journal.pone.0152075.
3. Sosič Izidor, Anderluh Marko, Sova Matej, Gobec Martina, Mlinarič-Raščan Irena, Derouaux Adeline, Amoroso Ana, Terrak Mohammed, Breukink Eefjan, Gobec Stanislav. Structure-activity relationships of novel tryptamine-based inhibitors of bacterial transglycosylase. Journal of Medicinal Chemistry 2015, 58, 9712-9721, doi: 10.1021/acs.jmedchem.5b01482.
4.Hrast Martina, Sosič Izidor, Šink Roman, Gobec Stanislav. Inhibitors of the peptidoglycan biosynthesis enzymes MurA-F. Bioorganic chemistry 2014, 55, 2-15, doi: 10.1016/j.bioorg.2014.03.008.
5. Hrast Martina, Anderluh Marko, Knez Damijan, Randall Christopher P., Barreteau Hélène, O'Neill Alex J., Blanot Didier, Gobec Stanislav. Design, synthesis and evaluation of second generation MurF inhibitors based ona cyanothiophene scaffold. European Journal of Medicinal Chemistry 2014, 73, 83-96, doi: 10.1016/j.ejmech.2013.11.031.
DNA gyrase and topoisomerase IV
Project leader: prof. dr. Danijel Kikelj
Description: DNA gyrase and topoisomerase IV as vital ATP-dependent enzymes for DNA replication are attractive targets for antibacterial drug discovery. Both enzymes are interesting drug targets for discovery of antibacterial agents because their structural similarities offer possibility of dual targeting, which prolongs the onset of resistance. Based on our recently discovered Escherichia coli GyrB inhibitors, design, synthesis and evaluation of new potency- and ADMET-optimised inhibitors focusing on balanced GyrB/ParE inhibition to achieve broad-spectrum antibacterial activity and avoid resistance development is in progress.
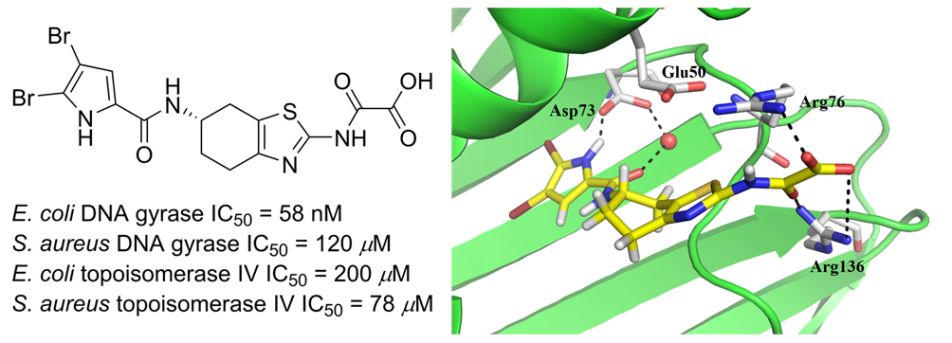
Novel 4,5,6,7-tetrahydrobenzo[1, 2-d]thiazole-based DNA gyrase inhibitors.
Recent publications:
1. Durcik, Martina, Nyerges, Ákos, Skok, Žiga, Gramec, Darja, Trontelj, Jurij, Zidar, Nace, Ilaš, Janez, Zega, Anamarija, Kikelj, Danijel, Peterlin-Mašič, Lucija, Tomašič, Tihomir, et al. New dual ATP-competitive inhibitors of bacterial DNA gyrase and topoisomerase IV active against ESKAPE pathogens. European Journal of Medicinal Chemistry 2021, 213, 113200 doi: 10.1016/j.ejmech.2021.113200.
2. Tomašič, Tihomir, Zubriené, Asta, Skok, Žiga, Martini, Riccardo, Pajk, Stane, Sosič, Izidor, Ilaš, Janez, Matulis, Daumantas, Bryant, Sharon D. Selective DNA gyrase inhibitors : multi-target in silico profiling with 3D-pharmacophores. Pharmaceuticals 2021, 14, 1-24.
3. Durcik, Martina, Skok, Žiga, Ilaš, Janez, Zidar, Nace, Zega, Anamarija, Szili, Petra, Draskovits, Gábor, Revesz, T., Kikelj, Danijel, Nyerges, Ákos, Pál, Csaba, Peterlin-Mašič, Lucija, Tomašič, Tihomir. Hybrid inhibitors of DNA gyrase A and B : design, synthesis and evaluation. Pharmaceutics 2021, 13, 1-17, doi: 10.3390/pharmaceutics13010006.
4. Skok, Žiga, Barančokova, Michaela, Benek, Ondřej, Durante Cruz, Cristina, Tammela, Päivi, Tomašič, Tihomir, Zidar, Nace, Peterlin-Mašič, Lucija, Zega, Anamarija, Kikelj, Danijel, Ilaš, Janez, et al. Exploring the chemical space of benzothiazole-based DNA gyrase B inhibitors. ACS Medicinal Chemistry Letters 2020, 11, 2433-2440, doi: 10.1021/acsmedchemlett.0c00416.
5. Nyerges, Ákos, Tomašič, Tihomir, Durcik, Martina, Revesz, T., Szili, Petra, Skok, Žiga, Zidar, Nace, Ilaš, Janez, Zega, Anamarija, Kikelj, Danijel, Peterlin-Mašič, Lucija, et al. Rational design of balanced dual-targeting antibiotics with limited resistance. PLoS Biology 2020, 18, 1-31, 10.1371/journal.pbio.3000819.
InhA
Project leader: prof. dr. Stanislav Gobec
Description: As a continuation of the work performed during EU FP7 ORCHID project (coordinator GlaxoSmithKline), we focus on the development of structurally novel inhibitors of Mycobacterium tuberculosis enoyl acyl carrier protein reductase (InhA) that facilitates reduction of long chain fatty acids. Strategies for the discovery of inhibitors are based on the available InhA crystal structures and encompass de-novo structure-based design, virtual screening, synthesis of inhibitors, their biochemical and structural analysis, antimicrobial, and toxicological evaluation.
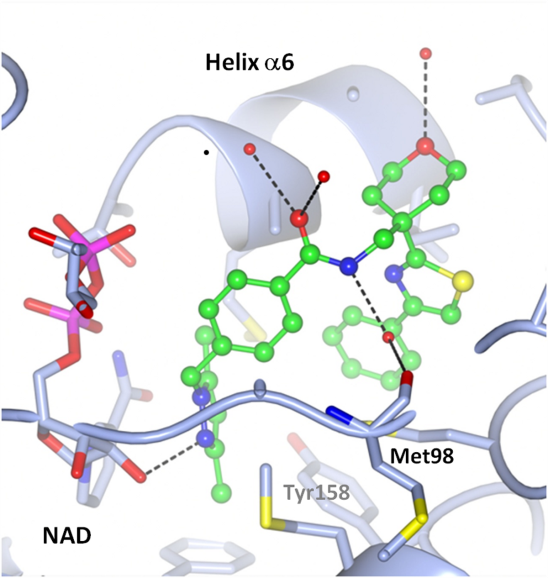
Co-crystal structure of a tetrahydropyran-based inhibitor in the InhA active site
Recent publications:
1. Štular Tanja, Lešnik Samo, Rožman Kaja, Schink Julia, Zdouc Mitja, Ghysels An, Liu Feng, Aldrich Courtney C., Haupt V. Joachim, Salentin Sebastian, Daminelli Simone, Schroeder Michael, Langer Thierry, Gobec Stanislav, Janežič Dušanka, Konc Janez. Discovery of Mycobacterium tuberculosis InhA inhibitors by binding sites comparison and ligands prediction. Journal of medicinal chemistry 2016, 59, 11069-11078.
2. Rožman Kaja, Sosič Izidor, Fernandez-Menendez Raquel, Young Robert J., Mendoza-Losana Alfonso, Gobec Stanislav, Encinas Lourdes. A new 'golden age' for the antitubercular target InhA. Drug Discovery Today 2016, 1-29, doi: 10.1016/j.drudis.2016.09.009.
3. Pajk Stane, Živec Matej, Šink Roman, Sosič Izidor, Neu Margarete, Chung Chun-Wa, Martínez-Hoyos María, Pérez-Herrán Esther, Álvarez-Gómez Daniel, Álvarez-Ruíz Emilio, Mendoza-Losana Alfonso, Castro-Pichel Julia, Barros David, Ballell-Pages Lluís, Young Robert J., Convery Maire A., Encinas Lourdes, Gobec Stanislav. New direct inhibitors of InhA with antimycobacterial activity based on a tetrahydropyran scaffold. European Journal of Medicinal Chemistry 2016, 112, 252-257, doi: 10.1016/j.ejmech.2016.02.008.
4. Šink Roman, Sosič Izidor, Živec Matej, Fernandez-Menendez Raquel, Turk Samo, Pajk Stane, Alvarez-Gomez Daniel, Lopez-Roman Eva Maria, Gonzalez Cortes Carolina, Rullas-Trincado Joaquin, Angulo-Barturen Iñigo, Barros David, Ballell Pages Lluís, Young Robert J., Encinas Lourdes, Gobec Stanislav. Design, synthesis and evaluation of new thiadiazole- based direct inhibitors of enoyl acyl carrier protein reductase (InhA) for the treatment of tuberculosis. Journal of Medicinal Chemistry 2015, 58, 613-624, doi: 10.1021/jm501029r.
FimH
Project leader: prof. dr. Marko Anderluh
Description: FimH, a a mannose-binding lectin is a crucial virulence factor for Escherichia coli urinary tract infections initiated by biofilm formation via the interaction of FimH with mannosylated bladder receptors. Blocking of FimH – mannosylated receptor interaction by small-molecule FimH antagonists is a promising approach to prevent E. coli urinary tract infections. Several groups reported α-D-mannosyl glycoconjugates as FimH adhesion inhibitors. Since no such molecule entered clinical studies, our aim is discovery of new FimH antagonists as antibacterial agents.
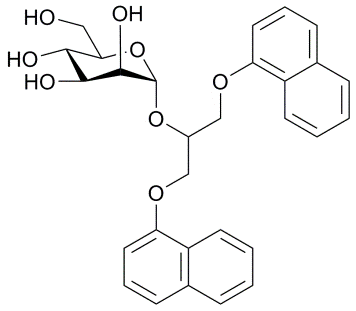
New α-D-mannopyranosides as FimH antagonists.
Recent publications:
1. Tomašič Tihomir, Rabbani Said Rahnamaye, Jakob Roman P., Reisner Andreas, Jakopin Žiga, Maier Timm, Ernst Beat, Anderluh Marko. Does targeting Arg98 of FimH lead to high affinity antagonists?. European Journal of Medicinal Chemistry 2021, 211, 1-16. doi: 10.1016/j.ejmech.2020.113093.
2. Tomašič Tihomir, Rabbani Said Rahnamaye, Gobec Martina, Mlinarič-Raščan Irena, Podlipnik Črtomir, Ernst Beat, Anderluh Marko. Branched α-D-mannopyranosides; a new class of potent FimH antagonists. MedChemComm 2014, 5, 1247-1253, doi: 10.1039/C4MD00093E.
2) Targeting immunoproteasome, NOD- and Toll-like receptors for discovery of immunomodulators
NOD receptors
Project leader: Assoc. Prof. Dr. Žiga Jakopin
Description: Intracellular nucleotide-binding oligomerization domain (NOD) receptors play an important role in pathogen recognition as part of the innate immune system. The target of muramyl dipeptide (MDP), the smallest immunostimulatory fragment of bacterial peptidoglycan, was identified as NOD2 receptor on which MDP exerts agonistic activity. NOD agonists are therefore potential immunostimulatory and indirect antibacterial agents. Small bacterial peptidoglycan fragments, D-Glu-meso-diaminopimelic acid (DAP) and MDP, are natural ligands of NOD1 and NOD2. Recently we have discovered immunostimulatory MDP analogues with NOD2 agonistic activity and D-Glu-meso-DAP-based NOD1 agonists. In continuation we plan to discover novel NOD1 in NOD2 agonists, employing the concept of peptido- and glycomimetics.
Recent publications:
1. Keček Kaja, Urbančič Dunja, Gobec Martina, Pekošak Aleksandra, Tomašič Tihomir, Anderluh Marko, Mlinarič-Raščan Irena, Jakopin Žiga. Identification of indole scaffold-based dual inhibitors of NOD1 and NOD2. Bioorganic & Medicinal Chemistry 2016, 24, 5221-5234, doi: 10.1016/j.bmc.2016.08.044.
2. Gobec Martina, Mlinarič-Raščan Irena, Sollner Dolenc Marija, Jakopin Žiga. Structural requirements of acylated Gly-l-Ala-d-Glu analogs for activation of the innate immune receptor NOD2. European Journal of Medicinal Chemistry 2016, 116, 1-12, doi: 10.1016/j.ejmech.2016.03.030.
3. Jakopin Žiga. Nucleotide-binding oligomerization domain (NOD) inhibitors: a rational approach toward inhibition of NOD signaling pathway. Journal of Medicinal Chemistry 2014, 57, 6897-6918, doi: 10.1021/jm401841p.
4. Jakopin Žiga, Gobec Martina, Kodela Jaka, Hazdovac Toni, Mlinarič-Raščan Irena, Sollner Dolenc Marija. Synthesis of conformationally constrained [gamma]-D-glutamyl-meso -diaminopimelic acid derivatives as ligands of nucleotide-binding oligomerization domain protein 1 (Nod1). European Journal of Medicinal Chemistry 2013, 69, 232-243. doi: 10.1016/j.ejmech.2013.08.022.
5. Jakopin Žiga, Gobec Martina, Mlinarič-Raščan Irena, Sollner Dolenc Marija. Immunomodulatory properties of novel nucleotide oligomerization domain 2 (Nod2) agonistic desmuramyldipeptides. Journal of Medicinal Chemistry 2012, 55, 6478-6488, doi: 10.1021/jm300503b.
Toll-like receptors
Project leader: prof. dr. Stanislav Gobec
Description: Toll-like receptors (TLR) represent the key component of human innate immune system,. They recognize specific structurally diverse molecular patterns, such as molecular components of microorganisms or endogenous molecules resulted from tissue damage. Up to date, 13 different mammalian TLRs have been reported, 10 of which have been identified as functional TLRs in humans. TLRs represent interesting target for therapeutic intervention of cancer, bacterial or viral infections and sepsis, rheumatoid artritis and several autoimmune diseases. Our research is focused on the search of novel modulators, agonists and/or antagonists of TLR4, TLR7 and TLR8. The compounds of interest will be designed by advanced in silico drug design approaches. New TLR modulators or IDO1 inhibitors will be designed with molecular modelling and docking software (in silico design) on the basis of crystallographic structural data for receptor and/or structures of ligands. Structure- and ligand-based virtual screening has elready lead to the discovery of TLR4 antagonists. Currently, the design, synthesis and evaluation of novel TLR7 and TLR8 agonist as potential immunomodulatory agents for cancer treatment are in progress
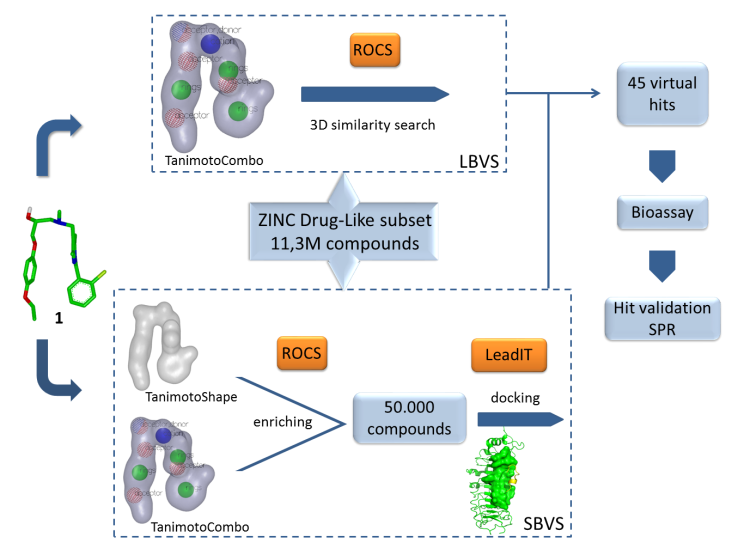
Design of novel TLR4 anatgonists and predicted binding position of hit compound (cyan) on TLR4 (green) by molecular docking study.
Recent publications:
1. Sova Matej, Rožman Kaja, Švajger Urban, Rožman Primož, Gobec Stanislav. Synthesis and biological evaluation of N-aryl-N'-(5-(2-hydroxybenzoyl)pyrimidin-2-yl)guanidines as toll-like receptor 4 antagonists synthesis of guanidine-based TLR4 antagonists. Medicinal Chemistry 2016, 12, doi: 10.2174/1573406412666160314151900.
2. Švajger Urban, Horvat Žiga, Knez Damijan, Rožman Primož, Turk Samo, Gobec Stanislav. New antagonists of toll-like receptor 7 discovered through 3D ligand-based virtual screening. Medicinal Chemistry Research 2015, 24, 362-371, doi: 10.1007/s00044-014-1127-5.
3. Švajger Urban, Brus Boris, Turk Samo, Sova Matej, Hodnik Vesna, Anderluh Gregor, Gobec Stanislav. Novel toll-like receptor 4 (TLR4) antagonists identified by structure- and ligand-based virtual screening. European Journal of Medicinal Chemistry 2013, 70, 393-399, doi: 10.1016/j.ejmech.2013.10.019.
Immunoproteasome
Project leader: prof. dr. Stanislav Gobec
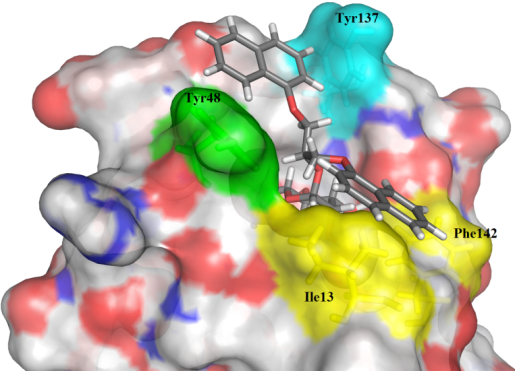
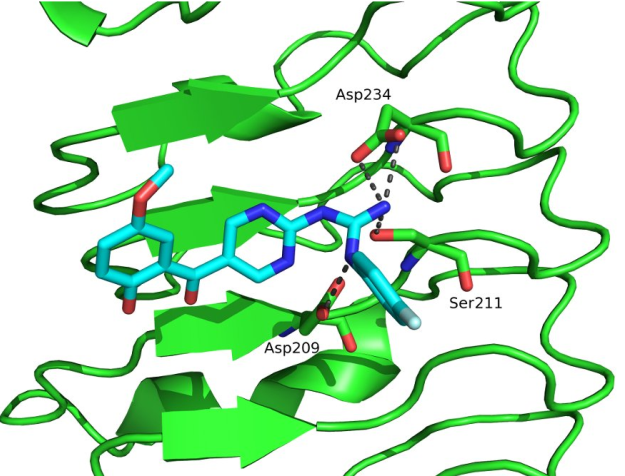
Description: In the vertebrates, the elevated expression of immunoproteasome is involved autoimmune, neurodegenerative, and some malignant diseases. The majority of known immunoproteasome inhibitors are nonspecific, as they inhibit also the constitutive proteasome. Therefore, it is postulated that immunoproteasome-specific inhibitors should show less side effects. Recently published crystal structure of the immunoproteasome is used as a basis for different (virtual) structure-based campaigns to discover selective immunoproteasome inhibitors. All prepared compounds are evaluated biochemically (on an isolated enzyme) and in in vitro functional assays to determine their immunomodulatory properties.
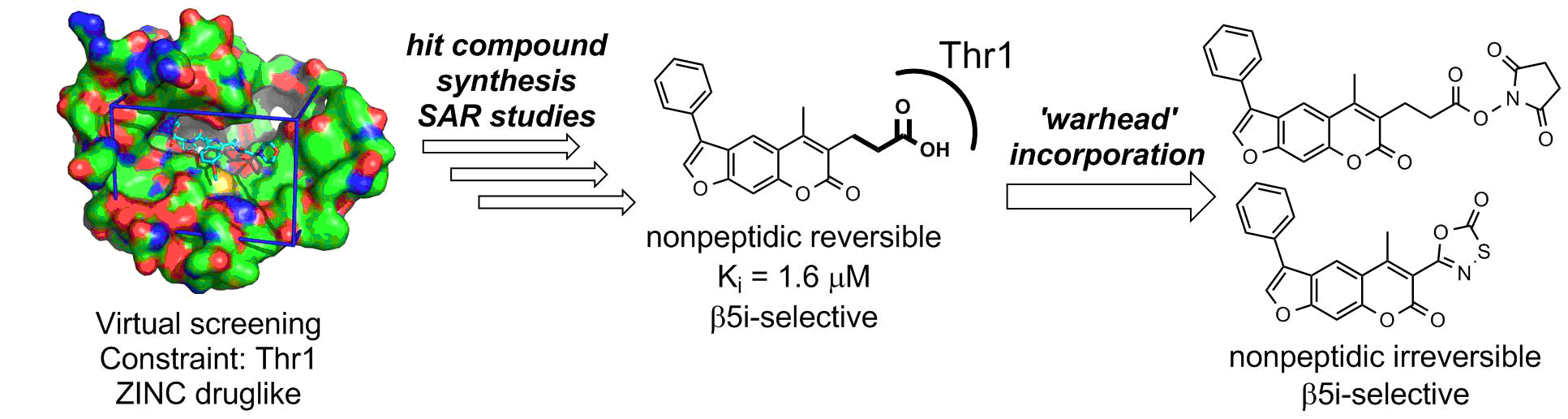
An example of a structure-based discovery and design of immunoproteasome-selective inhibitors.
Recent publications and patents:
1. Sosič Izidor, Gobec Martina, Brus Boris, Knez Damijan, Živec Matej, Konc Janez, Lešnik Samo, Ogrizek Mitja, Obreza Aleš, Žigon Dušan, Janežič Dušanka, Mlinarič-Raščan Irena, Gobec Stanislav. Nonpeptidic selective inhibitors of the chymotrypsin-like (ß5i) subunit of the immunoproteasome. Angewandte Chemie 2016, 55, 5745-5748, doi: 10.1002/anie.201600190.
2. Sosič Izidor, Gobec Martina, Brus Boris, Knez Damijan, Obreza Aleš, Mlinarič-Raščan Irena, Gobec Stanislav. Derivati psoralena kot nepeptidni zaviralci kimotripsinske aktivnosti imunoproteasoma: 24952 (A), 2016-09-30. Ljubljana: Urad Republike Slovenije za intelektualno lastnino.
3) Ion channels, choline esterases and amiloid beta aggregation as targets in the treatment of neurodegenerative diseases
Ion channels
Project leader: prof. dr. Danijel Kikelj
Description: Voltage-gated sodium (Nav) and potassium (Kv) channels are mainly found in excitable cells such as myocytes, endocrine cells and neurons of the central and peripheral nervous system, and are crucial for the initiation and propagation of electrical signals. The abnormally increased activity of Nav and Kv channels can cause neurodegenerative diseases, chronic pain, epilepsy, arrhythmias, and certain types of cancer. Structural information from the recently published crystal structures of bacterial Nav and Kv channels is a good starting point for design of selective Nav in Kv modulators. We have recently built human Nav homology model and prepared several classes of Nav modulators. Building on them, we design and synthesize new generations of improved analogues and evaluate them in electrophysiological assays on different Nav and Kv isoforms.
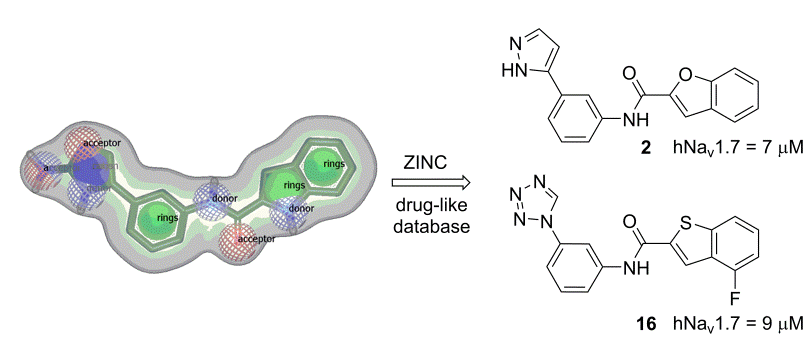
Discovery of novel voltage-gate sodium channel 1.7 blockers by virtual screening.
Recent publications:
1. Rogers Marc, Zidar Nace, Kikel, Danijel, Kirby Robert W. Characterization of endogenous sodium channels in the ND7-23 neuroblastoma cell line: implications for use as a heterologous ion channel expression system suitable for automated patch clamp screening. Assay and drug development technologies 2016, 14, 109-130, doi: 10.1089/adt.2016.704.
2. Jukič Marko, Frlan Rok, Chan Fiona, Kirby Robert W., Madge David J., Tytgat Jan, Peigneur Steve, Anderluh Marko, Kikelj Danijel. Synthesis and biological evaluation of piperazine derivatives as novel isoform selective voltage-gated sodium (Nav) 1.3 channel modulators. Medicinal Chemistry Research 2015, 24, 2366-2380, doi: 10.1007/s00044-014-1300-x.
3. Zidar Nace, Jakopin Žiga, Madge David J., Chan Fiona, Tytgat Jan, Peigneur Steve, Sollner Dolenc Marija, Tomašič Tihomir, Ilaš Janez, Peterlin Mašič Lucija, Kikelj Danijel. Substituted 4-phenyl-2-aminoimidazoles and 4-phenyl-4,5-dihydro-2-aminoimidazoles as voltage-gated sodium channel modulators. European Journal of Medicinal Chemistry 2014, 74, 23-30, doi: 10.1016/j.ejmech.2013.12.034.
4. Peigneur Steve, Žula Aleš, Zidar Nace, Chan-Porter Fiona, Kirby Robert, Madge David J., Ilaš Janez, Kikelj Danijel, Tytgat Jan. Action of clathrodin and analogues on voltage-gated sodium channels. Marine drugs 2014, 12, 2132-2143, doi: 10.3390/md12042132.
5. Tomašič Tihomir, Hartzoulakis Basil, Zidar Nace, Chan Fiona, Kirby Robert W., Madge David J., Peigneur Steve, Tytgat Jan, Kikelj Danijel. Ligand- and structure-based virtual screening for clathrodin-derived human voltage-gated sodium channel modulators. Journal of Chemical Information and Modeling 2013, 53, 3223-3232, doi: 10.1021/ci400505e.
Cholinesterase and monoamine oxidases
Project leader: prof. dr. Stanislav Gobec
Description: Butyrylcholinesterase (BChE) is a viable therapeutic target for restoring cholinergic activity and improving cognitive performance in Alzheimer’s disease (AD). Several other factors contribute to AD-associated neurodegeneration: aggregation and accumulation of amyloid β (Aβ), oxidative stress, loss of metal ion homeostasis, neuroinflammation and elevated activity of monoamine oxidases A and B (MAO-A and MAO-B). Additionally, MAO-B is a validated target in the therapy of other devastating neurodegenerative diseases, such as Parkinson’s disease. Recent observations also relate elevated MAO-A levels with pathologies such as cancer, cardiomyopathy and diabetes mellitus. With the re-emerging interest in these target enzymes, we have focused our research efforts on the discovery of selective BChE, MAO-A and MAO-B inhibitors as well as on multifunctional compounds targeting two or more targets. With multifunctional ligands we aim to counteract the deleterious effects of neuroinflammation and Aβ-cascade.
Two major series of selective BChE inhibitors with low nanomolar/picomolar inhibitory potencies were developed. Piperidine-based selective BChE inhibitors (see Figure) cross blood-brain barrier in vivo, and improve memory, learning capacity and cognitive functions of mice in scopolamine-induced model of cognitive impairment. Moreover, a sulfonamide-based selective BChE inhibitor markedly improved cognitive function and overall quality of life of dogs with canine cognitive dysfunction. Series of multifunctional ligands against AD based on the series of selective BChE inhibitors were also prepared. In collaboration with international partners, we developed a 11C PET tracer, evaluated its in vivo stability, biodistribution, and performed a longitudinal study of BChE activity in the brains of control and 5xFAD mice (model of AD) using PET in combination with computerized tomography. The results of the study unequivocally confirm that BChE is a promising biological marker for early form of AD. Secondly, potent tryptophan-derived BChE inhibitors were designed, synthesized and studied. The resolved X-ray structure of the inhibitors in complex with BChE enabled further optimization of on-target activities and improvement of ADMET properties. Safety and in vivo efficacy trial for lead tryptophan-based BChE inhibitor demonstrated a positive impact on memory retrieval without concomitant adverse motor effects. Lastly, a series of 1-propargyl-4-styrylpiperidine-like analogues we discovered represents a unique case of stereoselective activity of cis/trans isomers. Cis isomers are potent MAO-A inhibitors, whereas the trans analogues selectively target only the MAO-B isoform. Based on the positive results in vivo, BChE and MAO inhibitors represent an excellent starting point for further development of novel and safe agents against neurodegeneration.

Recent publications:
1. Maja Zakošek Pipan, Sonja Prpar Mihevc, Malan Štrbenc, Urban Košak, Ilija German Ilić, Jurij Trontelj, Simon Žakelj, Stanislav Gobec, Darja Pavlin, Gregor Majdič. Treatment of canine cognitive dysfunction with novel butyrylcholinesterase inhibitor. Scientific Reports. 2021, 11, 18098, doi: 10.7150/thno.54589. doi: 10.1038/s41598-021-97404-2.
2. Luka Rejc, Vanessa Gómez-Vallejo, Ana Joya, Óscar Moreno, Ander Egimendia, Pilar Castellnou, Xabier Ríos-Anglada, Unai Cossío, Urban Košak, Damijan Knez, Stanislav Gobec, Mariel Marder, Abraham Martin, Jordi Llop. Longitudinal evaluation of a novel BChE PET tracer as an early in vivo biomarker in the brain of a mouse model for Alzheimer disease. Theranostics. 2021, 11, 6542–6559, doi: 10.7150/thno.54589.
3. Anže Meden, Damijan Knez, Natalia Malikowska-Racia, Xavier Brazzolotto, Florian Nachon, Jurij Svete, Kinga Sałat, Uroš Grošelj, Stanislav Gobec. Structure-activity relationship study of tryptophan-based butyrylcholinesterase inhibitors. European Journal of Medicinal Chemistry. 2020, 208, 112766, doi: 10.1016/j.ejmech.2020.112766.
4. Urban Košak, Nika Strašek, Damijan Knez, Marko Jukič, Simon Žakelj, Abida Zahirović, Anja Pišlar, Xavier Brazzolotto, Florian Nachon, Janko Kos, Stanislav Gobec. N-Alkylpiperidine carbamates as potential anti-Alzheimer's agents. European Journal of Medicinal Chemistry. 2020, 197, 112282, doi: 10.1016/j.ejmech.2020.112282.
5. Damijan Knez, Natalia Colettis, Luca G. Iacovino, Matej Sova, Anja Pišlar, Janez Konc, Samo Lešnik, Josefina Higgs, Fabiola Kamecki, Irene Mangialavori, Ana Dolšak, Simon Žakelj, Jurij Trontelj, Janko Kos, Claudia Binda, Mariel Nora Marder, Stanislav Gobec. Stereoselective activity of 1-propargyl-4-styrylpiperidine-like analogues that can discriminate between monoamine oxidase isoforms A and B. Journal of Medicinal Chemistry 2020, 63, 1361–1387, doi: 10.1021/acs.jmedchem.9b01886.
6. Stane Pajk, Damijan Knez, Urban Košak, Maja Zorović, Xavier Brazzolotto, Nicolas Coquelle, Florian Nachon, Jacques-Philippe Colletier, Marko Živin, Jure Stojan, Stanislav Gobec. Development of potent reversible selective inhibitors of butyrylcholinesterase as fluorescent probes. Journal of Enzyme Inhibition and Medicinal Chemistry 2020, 35, 498–505, doi: 10.1080/14756366.2019.1710502.
7. Anže Meden, Damijan Knez, Marko Jukič, Xavier Brazzolotto, Marija Gršič, Anja Pišlar, Abida Zahirović, Janko Kos, Florian Nachon, Jurij Svete, Stanislav Gobec, Uroš Grošelj. Tryptophan-derived butyrylcholinesterase inhibitors as promising leads against Alzheimer's disease. Chemical Communications 2019, 55, 3765–3768, doi: 10.1039/C9CC01330J.
8. Urban Košak, Boris Brus, Damijan Knez, Simon Žakelj, Jurij Trontelj, Anja Pišlar, Roman Šink, Marko Jukič, Marko Živin, Adrian Podkowa, Florian Nachon, Xavier Brazzolotto, Jure Stojan, Janko Kos, Nicolas Coquelle, Kinga Sałat, Jacques-Philippe Colletier, Stanislav Gobec. The magic of crystal structure-based inhibitor optimization: development of a butyrylcholinesterase inhibitor with picomolar affinity and in vivo activity. Journal of Medicinal Chemistry 2018, 61, 119–139, doi: 10.1021/acs.jmedchem.7b01086.
4) Cathepsin B, pregnane X receptor and indolamine 2,3-dioxygenase as anticancer targets
Cathepsin B
Project leader: prof. dr. Stanislav Gobec
Description: Cathepsin B (CatB) participates in processes contributing to cancer progression. Recently, we have identified 5-nitro-8-hydroxyquinoline as a non-covalent, reversible and selective CatB inhibitor and prepared also more potent CatB inhibitors in a structure-based design campaign, based on a CatB inhibitor co-crystal structure, successfully resolved by us, that were able to reduce tumour progression through CatB inhibition. Based on the available 3D structural information, we have started rational design and targeted synthesis of next generation CatB inhibitors with improved properties. The inhibitors are being assessed both in functional tests that will reveal the effects of CatB inhibition on tumour progression and in in vivo mouse models. Concurrently, we are developing ruthenium-based complexes that possess derivatives 8-hydroxyquinoline as ligands. These metal-based compounds are assayed for their inhibition of CatB, followed by in vitro assays as inhibitors of extracellular matrix degradation.
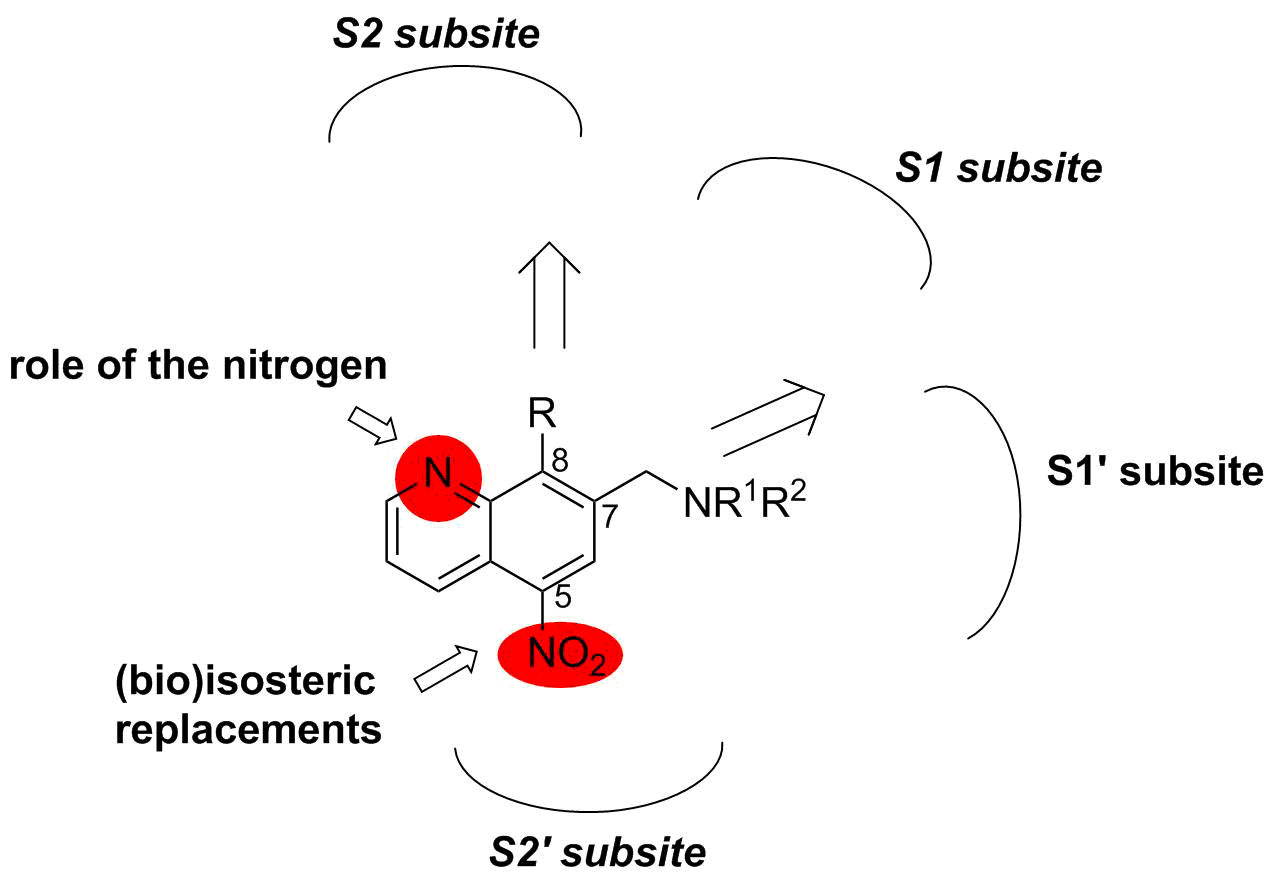
Schematic representation of modifications to nitroxoline.
Recent publications:
1. Mitrović Ana, Kljun Jakob, Sosič Izidor, Gobec Stanislav, Turel Iztok, Kos Janko. Clioquinol-ruthenium complex impairs tumour cell invasion by inhibiting cathepsin B activity. Dalton transactions 2016, doi: 10.1039/C6DT02369J.
2. Ogrizek Mitja, Turk Samo, Lešnik Samo, Sosič Izidor, Hodošček Milan, Mirković Bojana, Kos Janko, Janežič Dušanka, Gobec Stanislav, Konc Janez. Molecular dynamics to enhance structure-based virtual screening on cathepsin B. Journal of Computer-Aided Molecular Design 2015, 29, 707-712, doi: 10.1007/s10822-015-9847-2.
3. Mirković Bojana, Markelc Boštjan, Butinar Miha, Mitrović Ana, Sosič Izidor, Gobec Stanislav, Vasiljeva Olga, Turk Boris, Čemažar Maja, Serša Gregor, Kos Janko. Nitroxoline impairs tumor progression in vitro and in vivo by regulating cathepsin B activity. Oncotarget 2015, 6, 19027-19042.
4. Sosič, Izidor, Mirković, Bojana, Arenz, Katharina, Štefane, Bogdan, Kos, Janko, Gobec, Stanislav. Development of new cathepsin B inhibitors: combining bioisosteric replacements and structure-based design to explore the structure-activity relationships of nitroxoline derivatives. Journal of Medicinal Chemistry 2013, 56, 521-533, doi: 10.1021/jm301544x.
5. Mirković Bojana, Renko Miha, Turk Samo, Sosič Izidor, Jevnikar Zala, Obermajer Nataša, Turk Dušan, Gobec Stanislav, Kos Janko. Novel mechanism of cathepsin B inhibition by antibiotic nitroxoline and related compounds. ChemMedChem 2011, 6, 1351-1356, doi: 10.1002/cmdc.201100098.
Pregnane X receptor
Project leader: prof. dr. Danijel Kikelj
Description: The overexpression of pregnane X receptor (PXR) in cancer cells indicates its importance as a drug target for countering multidrug resistance in anticancer therapy. Inspired by marine steroids as natural leads and based on our preliminary results in MAREX project we have prepared novel steroidomimetic PXR modulators using ligand-based design approach, which are currently being optimised.
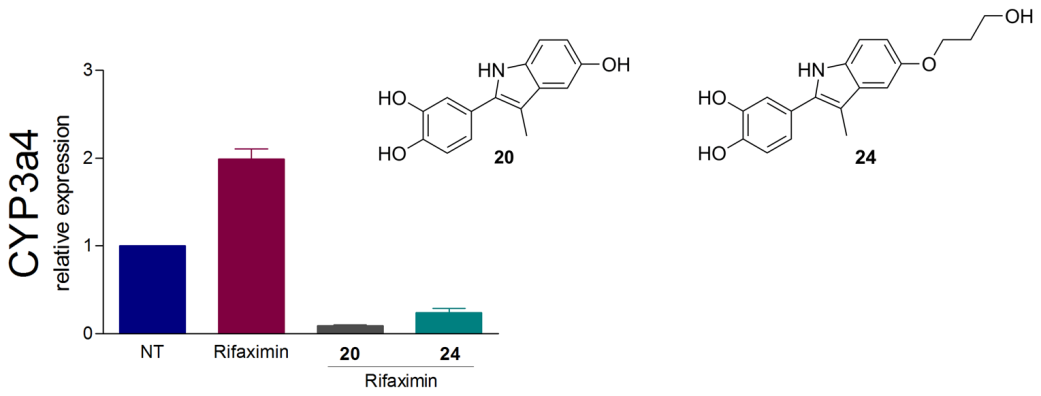
Solomonsterol A and B mimics based on the bazedoxifene scaffold as pregnane X receptor antagonists.
Recent publications:
1. Hodnik Žiga, Tomašič Tihomir, Smodiš Domen, D'Amore Claudio, Peterlin Mašič Lucija, Fiorucci Stefano, Kikelj Danijel. Diethylstilbestrol-scaffold-based pregnane X receptor modulators.
European Journal of Medicinal Chemistry 2015,
103, 551-562, doi: 10.1016/j.ejmech.2015.09.005.
2. Hodnik Žiga, Peterlin Mašič Lucija, Tomašič Tihomir, Smodiš Domen, D'Amore Claudio, Fiorucci Stefano, Kikelj Danijel. Bazedoxifene scaffold-based mimetics of solomonsterols A and B as novel pregnane X receptor antagonists.
Journal of Medicinal Chemistry 2014,
57, 4819-4833, doi: 10.1021/jm500351m.
Indolamine 2,3-dioxygenase
Project leader: Assist. Prof. Dr. Matej Sova
Description: The enzymes, involved in the kynurenine pathway of tryptophan metabolism, represent another interesting target for cancer immunotherapy. Indoleamine 2,3-dioxygenase 1 (IDO1) is a heme enzyme, which catalyzes the oxidation of L-tryptophan to N-formylkynurenine, which is known as the initial and rate limiting step of the kynurenine pathway. High IDO1 expression, which was found in tumor cells, triggers the escape from immune system and has been associated with poor prognosis in several cancer types. Thus, IDO1 activity is now considered as one of the most important factor in cancer proliferation and metastasis. Structure-based design will enable the discovery of novel IDO-1 inhibitors, which will be further on evaluated for potential anticancer effects.in cell-based functional tests.

Structure-based drug design of novel inhibitors,of indolamine 2,3-dioxygenase
Recent publications:
1. Dolšak Ana, Bratkovič Tomaž, Mlinarič Larisa, Ogorevc Eva, Švajger Urban, Gobec Stanislav, Sova Matej. Novel selective IDO1 inhibitors with isoxazolo[5,4-d]pyrimidin-4(5H)-one scaffold. Pharmaceuticals 2021, 14, 1-18, doi: 10.3390/ph14030265.
5) Custom synthesis of building blocks, biologically active small molecules, fluorescent labels and probes
Researchers at the Chair of pharmaceutical chemistry have long experience in the development of novel synthetic pathways and organic synthesis of small molecules (building blocks, intermediates and biologically active compounds). Compound classes belong to heterocycles, peptides, peptidomimetics, glycomimetics, steroidomimetics, lipidomimetics, natural products and their analogues, which are prepared using classical as well as modern organic synthesis methods (microwave-assisted, parallel, solid-phase synthesis). We also provide custom synthesis of fluorescent labels and probes (pH-sensitive probes, redox dependent probes, probes sensitive to the polarity of the environment, labels for polymers, membranes and proteins). Classical isolation and purification methods (TLC, column chromatography, HPLC) in combination with characterisation by spectroscopic methods (NMR, MS, IR, EPR, UV-VIS, fluorescence spectroscopy) are used to provide compounds of desired structure and purity.
a)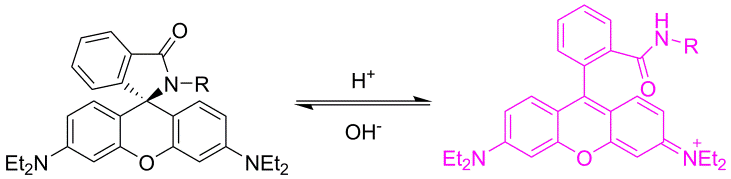 b)
b)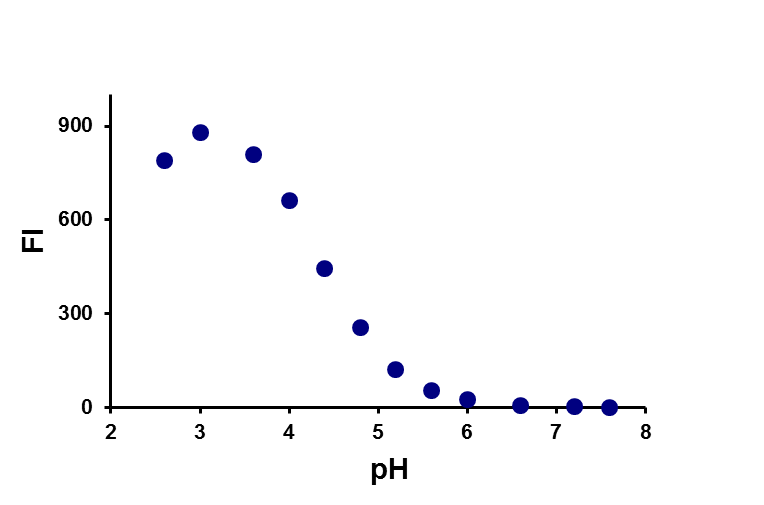
Example of a pH-dependent probe (a) and the effect of pH on fluorescence (b).
Recent publications and patents:
1. Sova Matej, Frlan Rok, Gobec Stanislav, Stavber Gaj, Časar Zdenko. D-Glucosamine in iron-catalysed cross-coupling reactions of Grignards with allylic and vinylic bromides: application to the synthesis of a key sitagliptin precursor. Applied organometallic chemistry 2015, 29, 528-535, doi: 10.1002/aoc.3327.
2. Žula Aleš, Kikelj Danijel, Ilaš Janez. A convenient strategy for synthesizing the Agelas alkaloids clathrodin, oroidin, and hymenidin and their (un)saturated linker analogs. Tetrahedron Letters 2014, 55, 3999-4001, doi: 10.1016/j.tetlet.2014.05.087.
3. Mravljak Janez, Ojsteršek Tadej, Pajk Stane, Sollner Dolenc Marija. Coumarin-based dual fluorescent spin-probes. Tetrahedron Letters 2013, 54, 5236-5238, doi: 10.1016/j.tetlet.2013.07.084.
4. Mravljak Janez, Sova Matej, Kovač Andreja, Gobec Stanislav, Časar Zdenko. Process for the synthesis of ezetimibe and intermediates useful therefor: patent no. JP5735913 (B2) - 2015-06-17. Tokio: Japan Patent Office, 2015. 28 str.
5. Živec Matej, Gobec Stanislav, Anžič Borut, Zupet Rok, Kolenc Ivanka. Novel process for synthesis of pramipexole and its pharmaceutically acceptable salts : CN101622235 (B), 2012-07-25: applicant: Krka tovarna zdravil, d.d. Bejing: Chinese Patent Office, SIPO, 2012. 18 str.
6) Toxicology in drug discovery
Description: Identification of metabolites is an important aspect at various stages of the drug development process. We will perform a combination of in silico prediction and in vitro GSH trapping assay using LCMS/MS for reactive metabolites determination. We have developed in silico and in vitro methods to verify endocrine disruptor (ED) potential of classes of compounds that may also affect the immune system. EDs, e.g. bisphenol A may modulate the endocrine system and other nuclear receptors by affecting the synthesis, transport, binding and elimination of endogenous hormones or by their epigenetic effect. We are trying to figure out the molecular mechanisms of these effects and evaluate if mixtures of EDs interact with each other to exhibit a synergistic effect.
Recent publications:
1. Gramec Darja, Schmidt Jan, Fic Anja, Klopčič Ivana, Trontelj Jurij, Sollner Dolenc Marija, Finel Moshe, Peterlin Mašič Lucija. Influence of metabolism on endocrine activities of bisphenol S. Chemosphere 2016, 157, 152-159, doi: 10.1016/j.chemosphere.2016.05.027.
2. Klopčič Ivana, Gramec Darja, Peterlin Mašič Lucija, Sollner Dolenc Marija. Comparison of in vitro hormone activities of novel flame retardants TBB, TBPH and their metabolites TBBA and TBMEPH using reporter gene assays. Chemosphere 2016, 160, 244-251, doi: 10.1016/j.chemosphere.2016.06.091.
3. Tišler Tatjana, Krel Alja, Gerželj Urška, Erjavec Boštjan, Sollner Dolenc Marija, Pintar Albin. Hazard identification and risk characterization of bisphenols A, F and AF to aquatic organisms. Environmental pollution 2016, 212, 472-479, doi: 10.1016/j.envpol.2016.02.045.
4. Gramec Darja, Tomašič Tihomir, Carino Adriana, Distrutti Eleonora, Fiorucci Stefano, Peterlin Mašič Lucija. New brominated flame retardants and their metabolites as activators of the pregnane X receptor. Toxicology Letters 2016, 259, 116-123, doi: 10.1016/j.toxlet.2016.08.005.
5. Fic Anja, Jurković Mlakar Simona, Juvan Peter, Mlakar Vid, Marc Janja, Sollner Dolenc Marija, Broberk Karin, Peterlin Mašič Lucija. Genome-wide gene expression profiling of low-dose, long-term exposure of human osteosarcoma cells to bisphenol A and its analogs bisphenols AF and S. Toxicology in vitro 2015, 29, 1060-1069, doi: 10.1016/j.tiv.2015.03.014.