Discovery and mechanism of action of novel hEag1 potassium channel lead molecules with anti-cancer activity
Code:
N1-0098
Range:
01. January 2019 - 31. December 2022
Range:
1,05 FTE
Leader:
Lucija Peterlin Mašič
Field:
1-09 Natural sciences and Mathematics - Pharmacy
Research Organisation:
https://cris.cobiss.net/ecris/si/en/project/17684
Researchers:
https://cris.cobiss.net/ecris/si/en/project/17684
Content:
https://cris.cobiss.net/ecris/si/en/project/17684
Abstract:
Cancer remains an important cause of mortality and economic losses worldwide. The Bilateral Project provides excellent research in new anticancer discovery for the treatment of gastrointestinal (GI) carcinomas (colorectal, gastric, liver and pancreatic cancers), where hEag1 potassium channels are overexpressed in 75 to 100% cases. GI carcinomas are among the 10 most-frequent forms of cancer in the EU. The added incidence of these four target cancers represents 24% of all cancers (WHO International Agency for Research on Cancer), with 640 000 new cases each year. Remarkably, the associated mortality is higher, at 370 000 deaths per year (29% of total), which reflects the bad prognosis with the current therapeutic options (GLOBOCAN 2012). The reasons for the unsatisfactory results in the management of each of these indications are not the same. Pancreas and liver cancers are among the most lethal oncological processes overall, and the present treatment strategies can only marginally increase patient survival. Gastric and colon adenocarcinomas are difficult to treat and require therapies that are an important burden to the patient, although the prognosis is not as bad as for pancreatic and liver cancers, which because of their high incidence, represent the second and third major causes of death by cancer worldwide. For an improved cancer therapy approach, a permanent need for the discovery of new-generation, refined anticancer agents is needed, as well as the elucidation of novel cancer targets.
These insights highlight the unmet medical need for development of new therapeutic strategies. Exploitation of genuinely novel classes of cancer targets with new mechanisms of action provides great opportunities for the discovery of new anticancer drugs for GI carcinomas. Targeting the voltage-gated potassium ion channels and hEAG1 in cancer is one of the most exciting recent advances in cancer biology. This Project is designed to discover novel hEAG1 inhibitors by targeting underexplored target with new mechanisms of action: voltage-gated potassium channels hEag1.
The Project aims to discover new selective small-molecule inhibitors of hEAG1 using drug-design methods and original platform for electrophysiological and anticancer evaluation. Proposed Project is an interdisciplinary research project between two partners and includes experises of both partners. The University of Ljubljana group is expert in computer-aided drug design methodologies and in organic synthesis. While the partner from the Max Planck Institute of Experimental Medicine (MPG), will integrate biological, genomic and molecular approaches with translational studies in different carcinomas. Prof. Luis Pardo is a pioneer in the research of the therapeutic potential of voltage-gated potassium channels in cancer. Complementary research include: understanding the genetic and biological basis of cancer, design strategies of new lead compounds, organic synthesis, innovative pharmacological evaluation, and hit-to-lead optimisation. The overarching goal is to discover first-in-class leads for hEag1 cancer target and to broaden treatment possibilities for different carcinomas.
The Consortium consists of excellent academic partner University of Ljubljana expert in medicinal chemistry and toxicology and the world-renowned cancer institute Max Planck Institute of Experimental Medicine (Germany), who will integrate biological, genomic and molecular approaches with translational studies in GI tumours. As the work will be performed within word-class European institutions that cover all of the important aspects of lead discovery, this will result in medically relevant research and in translation of the results into practice. This multidisciplinary Project will be of great interest for the pharmaceutical industry.
Cancer remains an important cause of mortality and economic losses worldwide. The Bilateral Project provides excellent research in new anticancer discovery for the treatment of gastrointestinal (GI) carcinomas (colorectal, gastric, liver and pancreatic cancers), where hEag1 potassium channels are overexpressed in 75 to 100% cases. GI carcinomas are among the 10 most-frequent forms of cancer in the EU. The added incidence of these four target cancers represents 24% of all cancers (WHO International Agency for Research on Cancer), with 640 000 new cases each year. Remarkably, the associated mortality is higher, at 370 000 deaths per year (29% of total), which reflects the bad prognosis with the current therapeutic options (GLOBOCAN 2012). The reasons for the unsatisfactory results in the management of each of these indications are not the same. Pancreas and liver cancers are among the most lethal oncological processes overall, and the present treatment strategies can only marginally increase patient survival. Gastric and colon adenocarcinomas are difficult to treat and require therapies that are an important burden to the patient, although the prognosis is not as bad as for pancreatic and liver cancers, which because of their high incidence, represent the second and third major causes of death by cancer worldwide. For an improved cancer therapy approach, a permanent need for the discovery of new-generation, refined anticancer agents is needed, as well as the elucidation of novel cancer targets.
These insights highlight the unmet medical need for development of new therapeutic strategies. Exploitation of genuinely novel classes of cancer targets with new mechanisms of action provides great opportunities for the discovery of new anticancer drugs for GI carcinomas. Targeting the voltage-gated potassium ion channels and hEAG1 in cancer is one of the most exciting recent advances in cancer biology. This Project is designed to discover novel hEAG1 inhibitors by targeting underexplored target with new mechanisms of action: voltage-gated potassium channels hEag1.
The Project aims to discover new selective small-molecule inhibitors of hEAG1 using drug-design methods and original platform for electrophysiological and anticancer evaluation. Proposed Project is an interdisciplinary research project between two partners and includes experises of both partners. The University of Ljubljana group is expert in computer-aided drug design methodologies and in organic synthesis. While the partner from the Max Planck Institute of Experimental Medicine (MPG), will integrate biological, genomic and molecular approaches with translational studies in different carcinomas. Prof. Luis Pardo is a pioneer in the research of the therapeutic potential of voltage-gated potassium channels in cancer. Complementary research include: understanding the genetic and biological basis of cancer, design strategies of new lead compounds, organic synthesis, innovative pharmacological evaluation, and hit-to-lead optimisation. The overarching goal is to discover first-in-class leads for hEag1 cancer target and to broaden treatment possibilities for different carcinomas.
The Consortium consists of excellent academic partner University of Ljubljana expert in medicinal chemistry and toxicology and the world-renowned cancer institute Max Planck Institute of Experimental Medicine (Germany), who will integrate biological, genomic and molecular approaches with translational studies in GI tumours. As the work will be performed within word-class European institutions that cover all of the important aspects of lead discovery, this will result in medically relevant research and in translation of the results into practice. This multidisciplinary Project will be of great interest for the pharmaceutical industry.
Cancer remains an important cause of mortality and economic losses worldwide. The Bilateral Project provides excellent research in new anticancer discovery for the treatment of gastrointestinal (GI) carcinomas (colorectal, gastric, liver and pancreatic cancers), where hEag1 potassium channels are overexpressed in 75 to 100% cases. GI carcinomas are among the 10 most-frequent forms of cancer in the EU. The added incidence of these four target cancers represents 24% of all cancers (WHO International Agency for Research on Cancer), with 640 000 new cases each year. Remarkably, the associated mortality is higher, at 370 000 deaths per year (29% of total), which reflects the bad prognosis with the current therapeutic options (GLOBOCAN 2012). The reasons for the unsatisfactory results in the management of each of these indications are not the same. Pancreas and liver cancers are among the most lethal oncological processes overall, and the present treatment strategies can only marginally increase patient survival. Gastric and colon adenocarcinomas are difficult to treat and require therapies that are an important burden to the patient, although the prognosis is not as bad as for pancreatic and liver cancers, which because of their high incidence, represent the second and third major causes of death by cancer worldwide. For an improved cancer therapy approach, a permanent need for the discovery of new-generation, refined anticancer agents is needed, as well as the elucidation of novel cancer targets.
These insights highlight the unmet medical need for development of new therapeutic strategies. Exploitation of genuinely novel classes of cancer targets with new mechanisms of action provides great opportunities for the discovery of new anticancer drugs for GI carcinomas. Targeting the voltage-gated potassium ion channels and hEAG1 in cancer is one of the most exciting recent advances in cancer biology. This Project is designed to discover novel hEAG1 inhibitors by targeting underexplored target with new mechanisms of action: voltage-gated potassium channels hEag1.
The Project aims to discover new selective small-molecule inhibitors of hEAG1 using drug-design methods and original platform for electrophysiological and anticancer evaluation. Proposed Project is an interdisciplinary research project between two partners and includes experises of both partners. The University of Ljubljana group is expert in computer-aided drug design methodologies and in organic synthesis. While the partner from the Max Planck Institute of Experimental Medicine (MPG), will integrate biological, genomic and molecular approaches with translational studies in different carcinomas. Prof. Luis Pardo is a pioneer in the research of the therapeutic potential of voltage-gated potassium channels in cancer. Complementary research include: understanding the genetic and biological basis of cancer, design strategies of new lead compounds, organic synthesis, innovative pharmacological evaluation, and hit-to-lead optimisation. The overarching goal is to discover first-in-class leads for hEag1 cancer target and to broaden treatment possibilities for different carcinomas.
The Consortium consists of excellent academic partner University of Ljubljana expert in medicinal chemistry and toxicology and the world-renowned cancer institute Max Planck Institute of Experimental Medicine (Germany), who will integrate biological, genomic and molecular approaches with translational studies in GI tumours. As the work will be performed within word-class European institutions that cover all of the important aspects of lead discovery, this will result in medically relevant research and in translation of the results into practice. This multidisciplinary Project will be of great interest for the pharmaceutical industry.
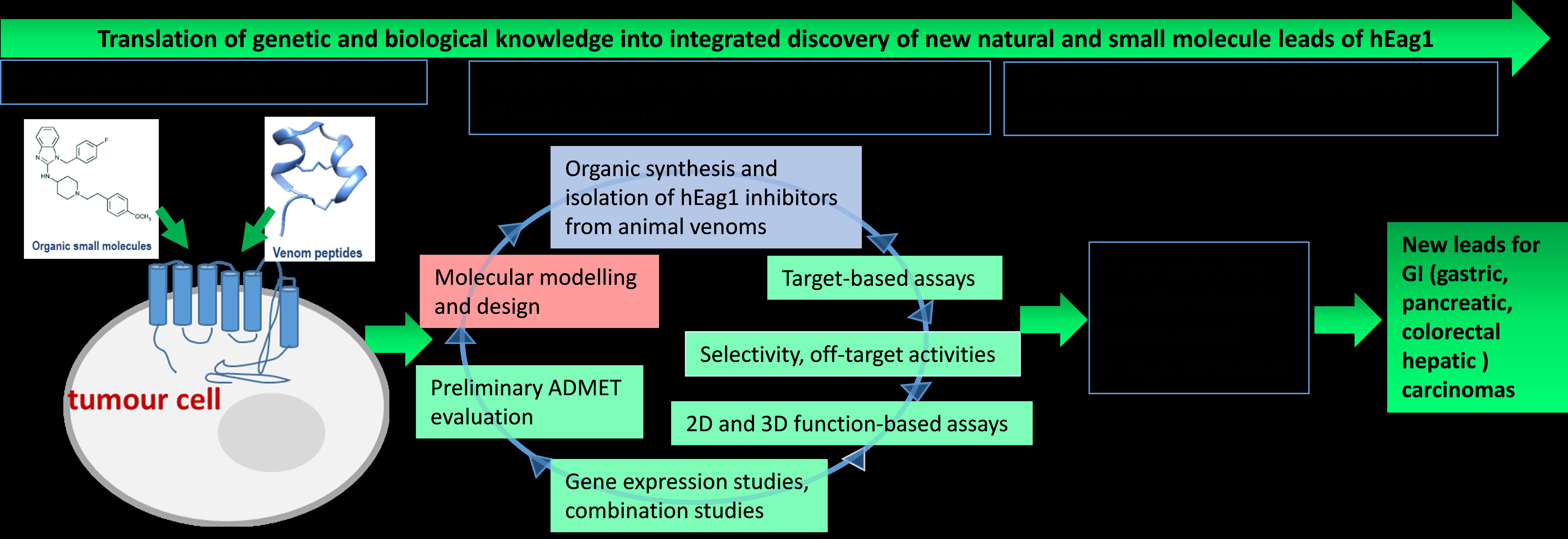
Presentation of the Project N1-0098 strategy to defeat cancer. hEag1 are voltage-gated potassium ion channels; ADMET: absorption, distribution, metabolism, elimination and toxicity; GI carcinomas: gastric, pancreatic, colorectal and hepatic.
Phases:
https://cris.cobiss.net/ecris/si/en/project/17684
Bibliographical references, arising directly from the implementation of the project:
https://cris.cobiss.net/ecris/si/en/project/17684
Financed by:

Research projects (co)funded by the Slovenian Research Agency.
Changed: 15. April 2020